- ISO 13485:2016 Compliant
Committed to Excellence in Outsourced Manufacturing for Medical Devices & Consumables


Manufactured in the USA
30+ Years of ISO 13485:2016 Manufacturing in the US
WHAT WE DO
- ISO 13485:2016 Manufacturing
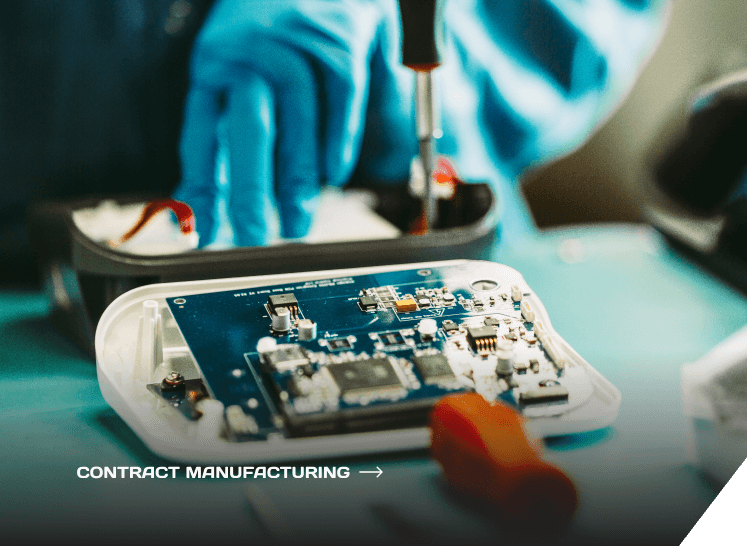
Contract Manufacturing for Medical Devices
Years of experience in ISO 13485:2016 manufacturing & engineering on a variety of platforms, systems integration, automation software and instrument development in the medical & diagnostics industries.
- Scalability, Volume & Automation
- Proven, World-Class Manufacturing above 97.5%
Our Clients Over the Years






From concept to delivery, we prioritize quality.
ISO 13485:2016

ISO 13485:2016 Manufacturing
We offer products that strictly follows the quality requirements of the ISO13485:2016 Quality Management System
Software Development
A team of experienced software developers that can help you with an application customized specifically for your hardware.
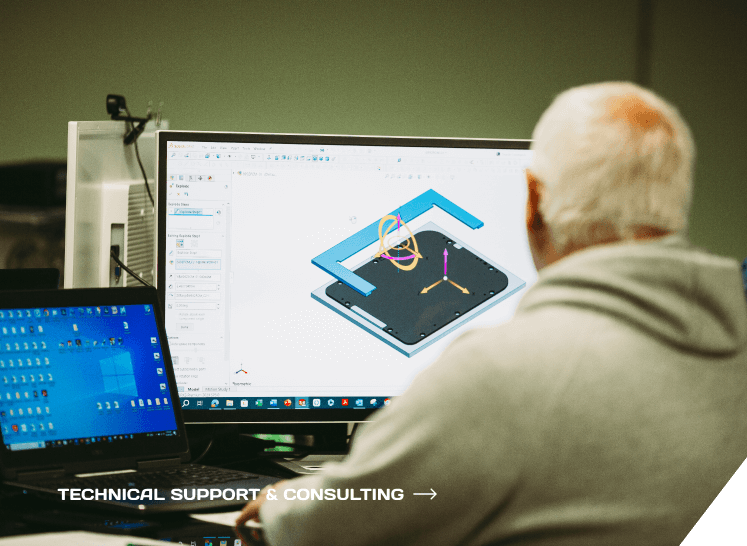
Engineering Consulting
We blend innovation, safety, and precision to create user-friendly point of care & medical devices for you.
About Calibrex Technologies
We combine conceptual creativity with state-of-the-art technological knowledge and expertise.
Markets we serve